2
MONOGENIC INHERITANCE:
OBJECTIVES:
·
By the end of this
session the student should be able to:
·
State the differences
between autosomal and X-linked, dominant and recessive inheritance
·
Give examples of diseases
showing different types of monogenic inheritance
·
Explain the meaning of new
mutations, penetrance and expressivity
·
Explain the importance of
consanguinity in autosomal recessive disorders
·
Calculate the expected frequencies of affected individuals and
carriers in monogenic diseases
·
Define the terms heterozygous, homozygous, and hemizygous
·
Explain why females are rarely affected by X-linked recessive
diseases
MONOGENIC INHERITANCE
This chapter
concerns the inheritance of characteristics that are determined by a single
gene pair. These are characteristics showing discontinuous
variation i.e. those represented by two or more contrasting
characteristics. There are few easily
visible, normal external characteristics that show monogenic inheritance.
Examples are eye colour, large or small ear lobes, sticky or dry earwax,
ability or inability to curl the tongue (tongue rolling), ability or inability
to taste the substance PTC, ability or inability to hyperextend the thumb
(hitch hiker's thumb). However, there are numerous examples of diseases that
follow a monogenic pattern of inheritance.
In humans, most
visible characteristics such as stature, blood pressure and intelligence show continuous variation. Each of these characteristics is represented
by a range of values. Such
characteristics are determined by several genes, which also interact with the
environment, and therefore show polygenic inheritance. For example stature is determined by a
number of inherited genes, but a high nutritional level during childhood also
contributes to increase stature.
Inherited genetic
diseases are examples of discontinuous variation. The disease is either present or is not, and there is no
intermediate range. They show monogenic
inheritance.
·
Genes
always go in pairs.
Alleles are the
possible alternative forms of the same gene.
The alleles in a gene pair may be identical or different.
The genotype is the
particular combination of genes in an individual.
The phenotype is the
sum total of the physical characteristics resulting from the expression of the
gene pair (genotype) in an individual.
In this chapter it
will be assumed that there are:
two possible alleles: A
a
three possible genotypes: A
A A a a a
![]()
two possible phenotypes:
dominant recessive
For each pair of
contrasting characteristics (phenotypes), one characteristic is dominant and
the other is recessive. The dominant characteristic is the one that
manifests itself in the heterozygote (Aa) as well as in the dominant homozygote (AA), whereas the recessive characteristic manifests itself only in
the recessive homozygote (aa).
AUTOSOMAL AND X-LINKED
INHERITANCE
· Autosomal inheritance applies to genetic disorders that are determined by a gene pair situated on the autosomes, i.e. the chromosomes other than the sex chromosomes X or Y, whereas X-linked disorders those that are determined by genes situated on the X chromosome. The main difference stems from the fact that there is only one X chromosome in males so that X-linked genes are unpaired in males, and paired in females. This gives rise to important differences in the transmission of X-linked diseases.
CRITERIA FOR AUTOSOMAL
DOMINANT INHERITANCE
An autosomal
dominant trait:
* is one that manifests itself in the
heterozygote
* is equally represented in males and females
* shows vertical inheritance through
generations i.e. transmission from parent to offspring
* affected parents
have 50 chance that their offspring will be similarly affected.
The outcome in the
offspring can be worked out using Punnett squares (a) if one parent is
affected; (b) if both parents are affected, as shown in the figures below.
|
(a) |
||
|
|
Affected Parent |
|
|
Normal Parent |
A |
a |
|
a |
A a |
a a |
|
a |
A a |
a a |
|
|
50% Affected |
50% Normal |
|
(b) |
||
|
|
Affected
Parent |
|
|
Affected
Parent |
A |
a |
|
A |
A
A |
A
a |
|
a |
A
a |
a
a |
|
|
75% Affected |
25%
Normal |
A dominant
homozygote (AA) can only result from two affected parents. As these diseases are rare, two parents
affected by the same autosomal dominant disorder would be extremely rare, and
so the dominant homozygote is practically never seen. Now, you can work out the outcome for the offspring of an
individual who is a dominant homozygote (AA) and a heterozygote (Aa)
partner.
Families with an autosomal dominant disorder usually show a
characteristic pedigree with vertical transmission from parents to offspring and affecting
both sexes. The arrow indicates the propositus, the
individual who presented for investigation.
EXAMPLES OF AUTOSOMAL DOMINANT DISORDERS
Achondroplasia: a type of
dwarfism characterised by short limbs, a normal-sized trunk and various skeletal
abnormalities.
Adult
polycystic kidney disease: multiple cysts in the kidneys and liver
causing symptoms and complications in adult life and leading to progressive
renal failure;
Brachydactyly:
meaning short fingers or toes.
Usually, the middle phalanx is short.
There are several varieties. In most cases it causes no inconvenience to
affected individuals.
Congenital
Spherocytosis: the red blood cells are in the form of spheres
rather than flat biconcave discs; they cause anaemia.
Familial
Adenomatous Polyposis (FAP): numerous polyps in the large intestine
becoming cancerous in most cases.
Familial
Hyperlipidaemia: elevated blood levels of low-density lipoproteins predisposing to coronary heart
disease at a young age. This is the
commonest autosomal dominant disorder.
Huntington's
disease: a late-onset neurological disorder,
appearing usually around the age of 35 years and characterized by abnormal,
involuntary writhing movements (chorea) and behaviour and personality
disorders. It is a severely
incapacitating and progressive disorder resulting in early death.
Marfan's
syndrome: a disorder of connective tissue. It causes abnormalities of the skeleton
including tall stature, wide arm span, spidery fingers and deformities of the
sternum; cardiovascular abnormalities such as aortic aneurysm or heart valve
incompetence; and subluxation of the lens.
Neurofibromatosis:
characterized by numerous cafe-au-lait pigmented patches and multiple benign
tumours of the nerve sheaths (neurofibromas) causing irregular swellings in the
skin
Postaxial
Polydactyly: characterised by an extra digit on the side of the little finger or
little toe, which may vary from a small tag to a well-developed digit. The extra digit is usually removed in early
infancy and forgotten.
Tuberous
sclerosis (adenoma sebaceum): characterised by an adenomatous rash on the cheeks,
calcifications in the brain and fits.
SITUATIONS THAT MAY MODIFY THE PATTERN OF AUTOSOMAL DOMINANT INHERITANCE
Some autosomal dominant disorders do not always
appear to follow the general rules stated above. Some cases do not have the
characteristic pedigree and have a negative family history. This may be due to
one of the following phenomena:
1. Conditions that manifest themselves late in life.
The typical example is Huntington's disease.
Although the gene for Huntington's disease is present from the time of
conception, affected individuals are normal until symptoms appear, usually
around the age of 45 years. Many individuals who carry the abnormal gene marry
and have children before the disease appears. The age at onset of the disease
is very variable ranging from 20 to 70 years.
Some individuals who have the abnormal gene might die from unrelated
causes before the disease appears. Thus
the pedigree may show an unaffected person who has affected offspring.
2. New Mutations
Autosomal dominant conditions
that have a negative family history are usually the results of new mutations. Individuals
with a severe disorder such as achondroplasia often have reduced chances of
getting married and having children, because of medical or social reasons. Achondroplastic
dwarfs have 50% of their children similarly affected. Most cases of achondroplasia, however, have
normal parents. These cases are the result of new mutations. For
a normal couple who had a child with achondroplasia arising as a new mutation,
there would be no increased recurrence risk for their other children. However,
the affected child would have a 50% chance of having affected offspring.
·
In general the more severe the
disease, the greater is the proportion of cases arising as new mutations
because the chances of affected individuals reproducing would be very
small. The following are a few
examples.

3. Variable Expressivity
There is often a considerable variation in the
severity of the disorder or in the type of abnormalities present although the
genetic defect is the same. This is
referred to as variable expressivity. Most genetic diseases show some degree of
variable expressivity. For example, in
postaxial polydactyly the extra digit may vary from a small skin tag to a fully
formed digit. In Marfan's syndrome affected individuals often do not
have all the characteristics - one individual may present with tall stature and
subluxated lens, another with an aortic aneurysm.
Sometimes the variability may be so great that
there may be little resemblance in phenotypic manifestations of an affected
individual and his affected offspring.
For example, in one type of limb reduction deformity the disorder may be
expressed to variable degrees from a missing finger to a missing leg.
4. Penetrance
In some cases individuals who carry an abnormal
gene do not appear to manifest any symptoms of the disease, although their
offspring may be affected and it would appear in the pedigree that the disease
has "skipped a generation".
The reason for this could be reduced penetrance.
Penetrance is a measure of how frequently a gene is expressed. It is the
proportion of heterozygotes who manifest the disorder. Some autosomal dominant
conditions e.g. achondroplasia have 100% penetrance i.e. when the gene is
present the disease always express itself.
In other conditions there may be reduced penetrance. Retinoblastoma is an autosomal dominant condition
that has 85% penetrance i.e. only 85% of persons carrying the abnormal gene
show symptoms of the condition; the remaining 15% never develop retinoblastoma
but can still transmit the gene to their offspring. Penetrance is important in
the assessment of recurrence risks.
CRITERIA FOR AUTOSOMAL RECESSIVE CONDITIONS
- The condition manifests itself only in
homozygotes;
- The parents of an affected individual are both
heterozygous (carriers) but are phenotypically normal;
- The condition is often present among sibs but
is not usually transmitted through generations;
- The offspring of heterozygous parents have a 1
in 4 chance of being affected; the ratio of affected to normal offspring
is 1 : 3.
· The following are some examples of autosomal recessive diseases:
Albinism: There is
lack of pigment in the skin, hair and eyes due to a deficiency in one of the
enzymes (e.g. tyrosinase) that is necessary for the formation of melanin
pigment from tyrosine. Lack of pigment
may cause visual problems and sensitivity of the skin to sunlight.
Beta
Thalassaemia: The adult type of haemoglobin (Hb A) is not produced or is produced in
very small amounts due to a defect in the production of beta-globin. This
causes severe anaemia beginning in early childhood and requiring frequent blood
transfusions. The bone marrow increases greatly in amount, trying to compensate
for the anaemia, causing a thickening of the bones of the skull and face.
Congenital
Hypothyroidism: This is due to lack of secretion of the hormone
thyroxine. Severe mental retardation
results unless replacement therapy with thyroxine is started in early infancy.
Cystic
Fibrosis: A disorder in the secretions of glands affecting mainly the respiratory
system, pancreas and sweat glands; death in infancy may result from recurrent
severe pulmonary infections.
Galactosaemia: This is
due to lack of an enzyme required to metabolise galactose. High levels of
galactose are present causing mental retardation, cataracts and cirrhosis of
the liver. These consequences can be
prevented if the disorder is recognised and treated in early infancy using
special milk substitutes that do not contain galactose and lactose.
Gangliosidosis:
Deficiency of the enzyme beta galactosidase causes accumulation of ganglioside
in the tissues including the brain, bone marrow, liver and spleen; involvement
of the brain causes fits, severe mental deterioration. Death usually occurs in
infancy.
Phenylketonuria: A defect
in phenylalanine metabolism causing mental retardation unless a special diet is
started early enough in infancy.
Sickle
cell anaemia: A disorder of the haemoglobin molecule causing the red blood cells to
become sickle shaped and rupture under conditions of low oxygen tension. This results in severe haemolytic anaemia.
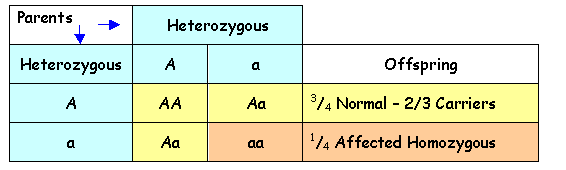
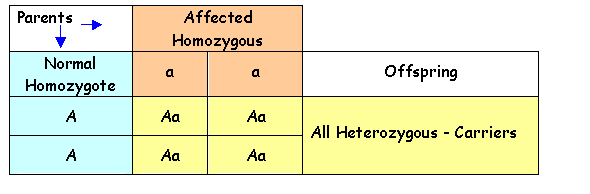
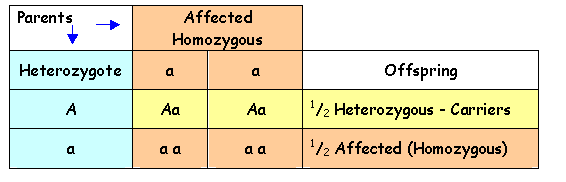
The following Punnett
Squares show the possible outcomes in the offspring of affected individuals and
carriers for autosomal recessive disorders.
Parents Normal
Homozygote Heterozygote A A Offspring- all
normal A AA AA 1/2 Homozygous - Normal a Aa Aa 1/2 Heterozygous - Carriers
a.
![]()
![]()
 b.
b.
c.
 d.
d.
The pedigree in
autosomal recessive disorders typically shows two or more affected sibs in
one family but other relatives are normal.
This is sometiomes called “horizontal” inheritance. In some cases the parents are consanguineous. First cousins have 1 in 8 of their genes in
common. Thus, if one individual were a
carrier (heterozygous) for an autosomal recessive disorder, the chances that
his or her first cousin is similarly affected would be 1 in 8. The risk for second cousins is 1 in 32, and
is less for more distant relationships.
However, in many cases of autosomal recessive disorder, the parents are
not related. This is especially so in
common disorders e.g. thalassaemia and
cystic
fibrosis. For rare disorders e.g. Wilson's disease cousin marriages would be more
frequent.
·
The following
pedigrees are commonly found in autosomal recessive inheritance (a) with
unrelated parents; (b) with consanguineous parents.
·
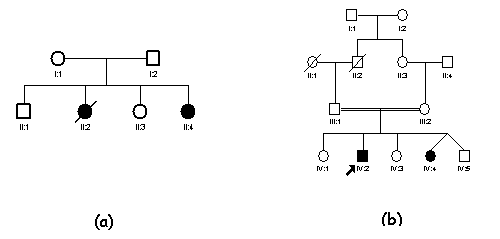
It is to be noted that affected individuals
arise only if both parents are heterozygous for the same gene.
This is quite obvious for most conditions e.g. if one parent is a
carrier (heterozygous) for B1 gangliosidosis and the other parent is a carrier
for phenylketonuria, there is no increased risk to the offspring. However, several different genes may cause
autosomal recessive congenital deafness.
If the parents were both carriers for the same gene, the risk for the offspring would be 1 in 4. However, if the parents are carriers for different recessive genes causing congenital deafness, there is no increased risk for
their offspring.
· In many cases there is only one affected child and the family history is negative. The pedigree in such cases would not be informative. The clinician usually identifies an autosomal recessive disorder from the clinical features and not from the pedigree. In small families in which the parents are both carriers for the same gene, there may be no affected individuals. Conversely, the greater the number of sibs, the more likely it is that at least one individual will be affected. In recent times most families have few children so that the typical pedigree with two or more affected sibs is becoming less common.
SEX-LINKED DISORDERS
X-LINKAGE
Sex-linked genes are those that are
situated on the sex chromosomes. Over
140 genes are X-linked. Very few of
these genes are related to sexual development. The vast majority are somatic
genes affecting various body functions such as blood clotting, colour vision,
body stature, and various metabolic processes. They are equally important in
males and females. There are very few
Y-linked genes and these are almost all related to male sexual development.
Obviously, Y-linked genes are found only in males. Because so few genes are located on the Y chromosome, the term
"sex-linked" is often taken to mean "X-linked" and the two
terms are used interchangeably.
Y-linked genes are important only in discussing sexual development.
We will take
haemophilia as an example of an X-linked recessive disorder. The abnormality is due to a defective gene
that produces factor VIII, one of the factors necessary for blood
coagulation.
We will call
the normal gene for factor VIII, X’, and its mutant allele responsible for
haemophilia Xh.
·
Females
have two X chromosomes and so three possible genotypes:
X'X' X'Xh XhXh.
· Males have only one X chromosome and so only two possible genotypes:
·
X' - Xh -.
The genotypes
for X-linked genes in males are termed hemizygous (Greek, hemi- = half). The terms homozygous and
heterozygous apply only for females. In
X-linked conditions heterozygous females (X'Xh) are carriers and
phenotypically normal; males may be either normal (X' -) or affected (Xh -) hemizygotes. The possible
offspring resulting from affected individuals and carriers are shown in the
Punnett Squares below.
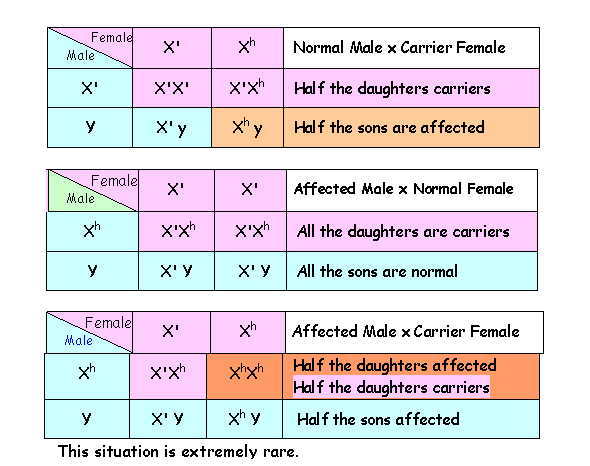
CRITERIA OF X-LINKED RECESSIVE INHERITANCE
· With few exceptions, the trait occurs only in males
·
The trait is transmitted from carrier females to affected males.
·
Male to male transmission excludes
X-linked inheritance.
The male offspring of an affected male individual are all normal; the female offspring are all carriers.
· An affected female is extremely rare and can only be the daughter of an affected male and a carrier female.
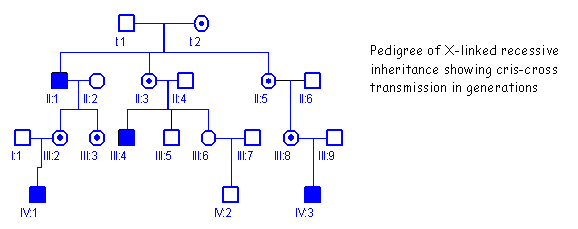
The
pedigree below is typical of X-linked recessive inheritance. The allele (X’) is transmitted from female
to male and male to female.
The trait
is expressed only in males and is transmitted by females.
It is often
described as a cris-cross pattern of inheritance because the disease appears in uncles and
nephews. The trait is not transmitted in direct lineage, but appears to
"skip a generation".
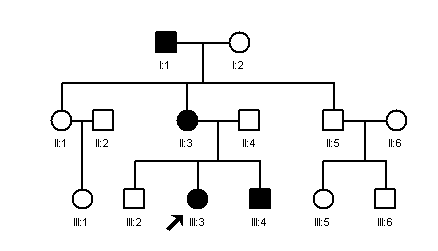
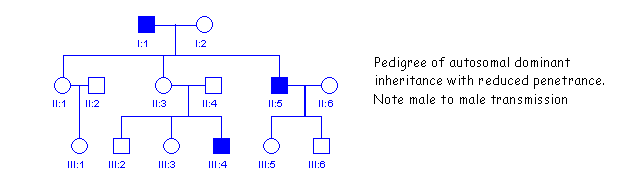
Note that the pedigree may be similar to that of autosomal dominant inheritance with reduced penetrance as is shown in the figure below. This also appears to skip a generation. Individual II3 in this pedigree must be heterozygous but does not show obvious symptoms of the disease. The distinguishing feature of theis pedigree is that it shows male to male transmission (I1 and II5), which is never seen in X-linked inheritance.
SOME X-LINKED TRAITS IN HUMANS
1. Haemophilia is characterised by inability to
form blood clots because of deficiency of factor VIII, a clotting factor in the blood. The frequency of haemophiliacs is about 1 in
10,000 males. Affected individuals
suffer from severe bleeding following a relatively small injury e.g. a cut or a
bruise. Bleeding into the joints may
occur leading to arthritis and joint stiffness. These complications may be avoided if affected individuals learn
how to self-administer factor VIII in case of injury and to keep a stock of it
at home.
2. Christmas disease is similar to Haemophilia but is
due to deficiency
of factor IX. The gene
for factor IX is also X-linked but is at a different locus from that of factor
VIII.
3. Duchenne Muscular
Dystrophy affects young
boys. It is characterised by muscular degeneration and weakness beginning at
the age of 3 to 5 years and progressing rapidly so that affected individuals
are wheel-chair bound by the time they are in their teens and die in their
early twenties because of severe involvement of their respiratory muscles.
There is deficiency of the protein dystrophin in skeletal muscle, caused by a mutation of the dystrophin gene.
4. Becker muscular dystrophy also affects males but muscular
weakness begins in adolescence, the condition progresses slowly and is not
lethal. It is also caused by a mutation
of the dystrophin
gene, the same gene
that produces Duchenne muscular dystrophy, but in this case the protein
dystrophin is only slightly abnormal.
5. Red-green colour
blindness is the
inability to distinguish red from green.
There are two adjacent genes on the X-chromosome, both related to colour
vision. One of theses genes produces a retinal pigment for red colour perception and the
other produces a pigment for green colour perception. A mutation affecting one or the other gene causes partial
red-green colour blindness while mutations affecting both genes causes total
red-green colour blindness.
6. Glucose-6-phosphate
dehydrogenase (G-6PD) deficiency is caused by deficiency of the enzyme G-6PD that plays a key role in
glucose metabolism. When persons with
this deficiency ingest certain drugs or foodstuffs, particularly the broad bean
vicia fava, there is haemolysis that causes anaemia. The symptoms of the condition can be
prevented if care is taken to avoid ingestion of the offending foods.
X-LINKED DOMINANT DISEASES
An example
is Vitamin
D-resistant rickets. This results from a defect in vitamin D
metabolism. Unlike the common type of rickets, it does not respond to
therapeutic doses of vitamin D. Being X-linked dominant, it affects
heterozygous females (X'Xh) as well as hemizygous males (Xh
-). The Punnett squares for X-linked
recessive traits shown above still apply with the difference that female
heterozygotes are affected. The pattern
of transmission is very similar to that of autosomal dominant conditions but
male-to-male transmission never occurs.
An affected mother will have half her sons and daughters affected. An affected male will have all his daughters
affected but all his sons will be normal.
THE LYON HYPOTHESIS: X- INACTIVATION
X-linked
genes are all paired in females but are present as single copies in males. This
implies that only a single copy of these genes is necessary for normal
function. One would expect that the proteins regulated by the X-linked genes
would be present in half the amounts in males as compared to females. However, this is not so. The amount of protein produced is
approximately equal in males and females.
The Lyon hypothesis, proposed by the geneticist Mary Lyon in 1961,
states that only one X chromosome is active in any cell and that any X
chromosomes in excess of one are inactivated.
The
phenomenon of X inactivation can be observed in the cells of females: the
inactivated X chromosome forms a tightly coiled clump of chromatin which is
visible in the nuclei of cells as a mass adjacent to the nuclear envelope and
known as a Barr body. As a result there is only one functional X chromosome in
both males and females. This phenomenon is known as dosage compensation.
The Lyon
hypothesis can be verified in individuals with numerical X chromosome
abnormalities. Males, who have 47, XXY
chromosomes (Klinefelter Syndrome) have one Barr body. Males with 48,XXXY
chromosomes and females with 47,XXX chromosomes (trisomy X) both have two Barr
bodies. In contrast females with Turner
Syndrome (45, X chromosomes) and normal males (46, XY) have no Barr bodies. It is now clear that monosomies, trisomies
and tetrasomies of the X chromosomes, are compatible with life because of the
phenomenon of X inactivation. However,
it is also clear that X-inactivation is only partial, since the persons
affected with the above mentioned sex-chromosome abnormalities have defects
affecting both somatic and sexual development.
X-INACTIVATION IS RANDOM.
Which of
the two X chromosomes is inactivated in females is purely random. Let us consider a particular locus on the X
chromosome, such as that of haemophilia (factor VIII) in a heterozygote
(carrier female). In about half the cells the X chromosome bearing the normal
allele would be inactivated and in the other half the X chromosome bearing the
mutant allele for haemophilia would be inactivated. Thus only half the cells would produce factor VIII and only half
the amount of factor VIII would be present in the circulation. However, this is
sufficient for normal blood clotting.
Random X
inactivation occurs during foetal development. In some cells, the maternally-
derived X chromosome is inactivated, while in others the paternally derived X
chromosome is inactivated. The mass of
cells, which arise by mitosis from one particular cell, is called a clone. After X inactivation during foetal life, all
the cells of one clone would have the same X chromosome inactivated.
Random X
inactivation can be observed dramatically in female "tortoise shell"
cats, which have patches of black fur and patches of rust coloured fur. The genes for black and rust hair pigment
are alleles situated on the X chromosome; we will these call Xb and
Xr respectively. In heterozygous females (Xb Xr )
some skin cells will have the Xb
allele inactivated and produce patches of rust coloured fur, while
others will have the Xr gene
inactivated and produce patches of black fur.
A similar
example of random X inactivation in humans is seen in X-linked ectodermal dysplasia.
Males having the mutant X-linked gene (Xm -) have no sweat
glands (with consequent heat intolerance), have scanty body hair, early
baldness and abnormal teeth. Females
heterozygous for the X-linked mutant allele (X' Xm ) have patches of
skin in which sweat glands are absent.
SEX-INFLUENCED INHERITANCE
Sex-influenced
conditions are to be distinguished from X-linked recessive conditions. The
genes responsible for sex influenced inheritance are autosomal but their
expression is influenced by sex. For example an autosomal dominant gene causes
male pattern baldness. The condition is
expressed only in males. Its expression
is dependent on the presence of high levels of testosterone. Females may also inherit the gene and
transmit it to their offspring but do they do not express the trait because
they have a low testosterone level. The trait is expressed only in males but,
unlike X-linked inheritance, male to male transmission occurs as well as
transmission through carrier females.
PROBLEMS
1. A man with red-green colour blindness is
married to a girl with normal colour vision.
What is the probability that his children will be colour blind?
2. A boy
has Duchenne muscular dystrophy. His maternal uncle had died of muscular
dystrophy at the age of 21 years. What
is the probability that this child's sibs will be affected?
3. The adjacent pedigree is of a family with
haemophilia. If the gene for
haemophilia is represented by Xh and the normal allele by X', write
the genotypes of each individual in the pedigree. If the genotype cannot be
determined with certainty write the possible alternatives.
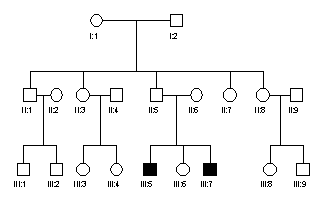
4. Explain,
giving reasons, whether the following pedigrees are compatible with autosomal
dominant, autosomal recessive or X-linked dominant and X-linked recessive
inheritance. (Note that a pedigree may
be compatible with more than one type of inheritance.)
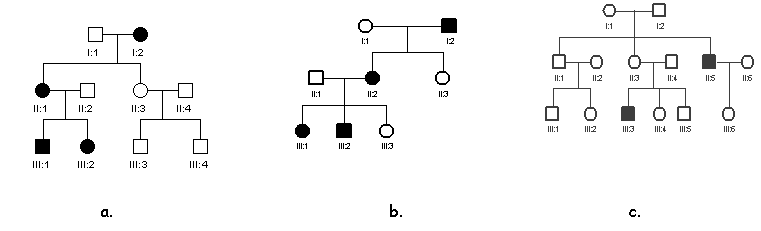
a.
b.
c.
5. Examine
the pedigree from a family with a genetic disease and answer the questions
below:
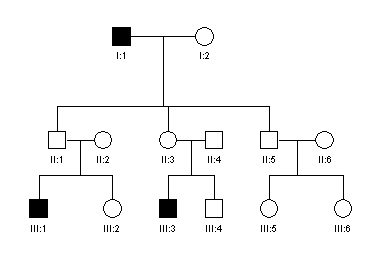
a. Does
this pedigree indicate autosomal dominant, recessive or sex-linked type of
inheritance? Give reasons for your
choice.
b. Assuming
that B and b are the normal and mutant alleles
respectively, what would be the genotypes of the individuals: II.1, II.2
and III.3 ?
c.
Individual II.3 requested
genetic counselling. What is the
probability that her child would be affected. Explain why.
6. A young
lady requested pre-marital genetic counselling because her sister had died in
infancy of gangliosidosis, an autosomal recessive disease. What is the risk
that this young lady has similarly affected offspring? What advice should be given?
7. A young
man requested pre-marital genetic counselling because his brother is an
achondroplastic dwarf. This condition
is inherited as an autosomal dominant disorder. What is the risk that his
offspring will be similarly affected?
What advice should be given?
8. A young couple had their first child affected
with cystic fibrosis. What is the risk
that their future children will be similarly affected?
*************************