The Extracellular Matrix
Professor Alfred Cuschieri
Department of Anatomy, University of Malta
Objectives
By the end of this session student should be able to:
·
Name the main extra cellular fibers and their locations
·
Outline the molecular composition of collagen fibers
·
Deduce the major consequences of defects in collagen formation on the functions of the extra cellular matrix
·
List the factors that could affect collagen synthesis and affect wound
healing
·
Name the non-fibrillar components of extra cellular fibers and their
functions
· Give examples of how cells interact with the extra cellular matrix
Recommended Reading
The World of the Cell.
Becker WM, Kleinsmith LJ, Hardin J. 5th Edition. Chapter 11
The Extracellular Matrix Of
Animal Cells p.290-301
The Extracellular Structures
All cells are associated with extra cellular structures. The connective tissues consist predominantly of extra cellular structures, which consist of:
·
Fibres - Collagen, elastic and fibrillin
·
Matrix (amorphous ground substance)
·
Fibroblasts and cells of various types
Collagen
Collagen is
a family of extra cellular fibres that are widely represented in the body and
have mainly mechanical functions. Collagen fibres provide strength and
support.
10 types of collagen are known. They vary in :
a. molecular structure
b. morphology
c. distribution
Molecular Structure
All collagens have a basically similar molecular structure:
They contain large amounts of:
o Glycine (about 30%)
Hydroxyproline and hydroxylysine (25%).
o (These amino acids
are rare in other proteins)
o Oligosaccharide
side chains (galactose and glucose)
Collagen is composed of molecules of pro-collagen. A pro-collagen molecule is composed of three polypeptide chains called a-chains, twisted in a helical fashion. The individual a-chains are termed pre-procollagen.
The general chemical
structure of pro-collagen molecules is shown in the following diagram. (G glycine; X proline or hydroxyproline; Y lysine or hydroxylysine; A
other amino acids)
G X A G A A G Y A G A A G X A G A
A G X A G A A G Y A G A A G X A G
A A G X A G A A G Y A G A A G X A
There are several different a-chains. They are found in different combinations in the different types of collagen, as shown below.
The
following table illustrates the molecular structure, morphology and
distribution of the first five types of collagen. The first five types are the most important ones. The other five are less important.
Collagen Type |
Morphology |
Molecular structure |
Distribution |
|
Type I |
Fibres with periodicity of 63 nm. ·
|
a1-1; a1-1; a1-2 low in carbohydrates |
·
Ligaments ·
Tendons ·
Fasciae ·
Bones ·
Dermis of skin ·
Sclera |
|
Type II |
Very thin fibrils forming a meshwork |
a1-2; a1-2; a1-2 high in hydroxylysine and carbohydrates
·
|
·
Hyaline cartilage ·
Cornea ·
Vitreous body |
|
Type III |
Loosely-packed network of thin fibrils |
a1-3; a1-3; a1-3 low in hydroxylysine · |
·
Associated with type 1 ·
Blood vessels ·
Most internal organs ·
Smooth muscle ·
Foetal dermis |
|
Type IV |
Thin amorphous membrane |
a1-4; a1-4; a1-4 Extensive hydroxylysine Heavy glycosylation
|
·
Basal laminae |
|
Type V |
Amorphous |
a1-5; a2-5; a3-5; high hydroxylysine; low alanine
·
|
·
Placenta ·
Foetal membranes ·
Skin ·
Blood vessels |
Biosynthesis of
Collagen
This involves a series
of sequential steps occurring in the rough endoplasmic reticulum and the Golgi
complex:
1. Synthesis of a chains of pre-procollagen on ribosomes. A signal protein directs them to the RER .
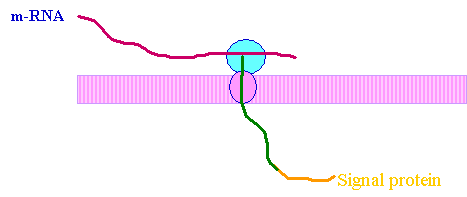
2. Cleavage of signal protein forms procollagen
3. Hydroxylation of lysine and proline
Lysine ΰ Hydroxylysine
Peptidyl lysine hydroxylase
Proline ΰ Hydroxyroline
Peptidyl proline hydroxylase

Ascorbic acid is necessary to activate the hydroxylases.
4.
Glycosylation: addition of galactose and glucose to some hydroxylysine residues. The enzymes galactosyl
transferase and glycosyl transferase are required for this process.
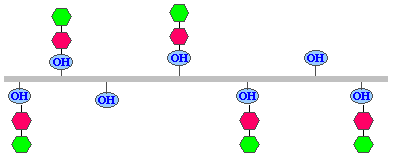
5. Assembly of three a - chains to form procollagen. This involves the formation of
disulphide bonds between parts of the polypeptide chains known as registration peptides, which occur at both ends of the
pre-procollagen.
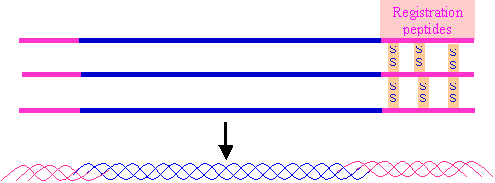
The assembly process aligns the three a - chains relative
to one another.
The three alpha chains are wound around one another in the form of a
triple helix.
The assembly process occurs in the Golgi apparatus.
6. Secretion of procollagen molecules by exocytosis into the
extra cellular space.
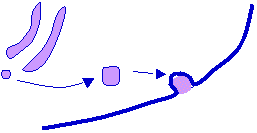
7. Cleavage of registration
peptides occurs in the extra cellular space, and is catalysed by procollagen
peptidases. The resulting molecule is
called tropocollagen.

8. Self-assembly or polymerization of tropocollagen molecules form collagen fibrils.
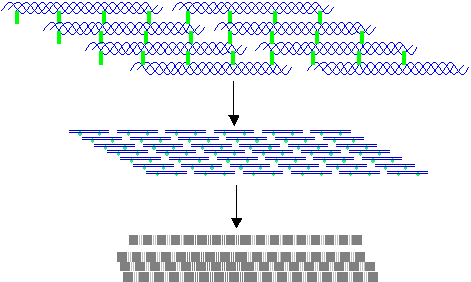 Cross-linkage between adjacent tropocollagen
molecules stabilizes the fibrils. This
occurs by oxidation deamination of the amino acid hydroxylysine. It involves the removal of an amino group
(NH2), which
has a net oxidative effect and the formation of covalent cross-links. It is catalyzed by lysine oxidase (or
catalase).
Cross-linkage between adjacent tropocollagen
molecules stabilizes the fibrils. This
occurs by oxidation deamination of the amino acid hydroxylysine. It involves the removal of an amino group
(NH2), which
has a net oxidative effect and the formation of covalent cross-links. It is catalyzed by lysine oxidase (or
catalase).
In collagen type 1 the tropocollagen
molecules polymerize in a staggered pattern, giving rise to long fibrils that,
under high magnification electron microscopy, appear to be striated fibers with
a repeat periodicity of 64 nm.
The fibers are usually arranged in
bundles that are visible with light microscopy.
Several connective tissue disorders are the result of mutations in the
alpha procollagen chains
Osteogensis imperfecta
Ontogenesis imperfecta (OI) is a defect in collagen type I. There are several types of OI, with somewhat different clinical manifestations. Most of them are caused by genetic mutations in the gene for the a 1-1 pre-procollagen.
The main clinical features involve poorly formed bone, which
results in:
o
Short, curved, and deformed bones
o
Multiple fractures, which are present at
birth in some cases
o
Abnormal dentine
o
Deafness due to defects in the small
ossicles of the ear
Ehlers Danlos Syndrome
There are several different types of Ehlers Danlos Syndrome. The following are a few common types to illustrate different molecular disorders in the formation of collagen.
Ehlers Danlos Syndrome Type I
This is caused by lack of synthesis or other defect of
pro a 2-1, resulting in poorly formed collagen type I. The main clinical features affect:
o
The skin is thin, hyperextensible, and easily bruised
o
Joint hypermobility
o
Anomalies of the heart valves, which are usually
incompetent
Ehlers Danlos Syndrome Type IV
This is caused by a defect of collagen type III. It affects mainly the collagen framework in the viscera, and may result in:
o Rupture of viscera e.g. bowel, arteries or uterus
o Thin, translucent (but not hyperextensible) skin
Ehlers Danlos Syndrome Type VI
This is caused by deficiency of lysine hydroxylase, the
enzyme responsible for hydroxylation of lysine. This affects the subsequent glycosylation and cross-linking. The main clinical manifestations are:
o Eye abnormalities abnormalities of sclera and cornea
o Hyperextensible joints
o Scoliosis
o Hyperextensible skin
Ascorbic Acid Deficiency (Scurvy)
This is
caused by a dietary deficiency of ascorbic acid. Although it is now rare, it was once common particularly among
sailors, and is still found in underdeveloped and undernourished populations. Ascorbic acid is necessary for activating
proline and lysine hydroxylases, resulting in poor hydroxylation of lysine and proline. It results in:
o Poor collagen fibre formation
o Poor wound healing
o Blood vessel fragility
o Skin lesions
o diseased gums
Ascorbic
acid is present in fresh fruit, and vegetables, and especially in citrus
fruit. The old custom of giving oranges
when visiting patients in hospital was intended to promote a speedy recovery,
but the cheerful colours of flowers appear to have become more popular!
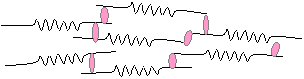
Elastin
Elastin consists of fibers, whose main property is their
elasticity. Elastic fibers are composed
of protein molecules with the following properties that differ from collagen in
many respects. It contains:
o
Abundant
glycine, about 33% (as in collagen)
o Little hydroxyproline
o No hydroxylysine
Abundant hydrophobic amino acids
Helical segments that are responsible for its elasticity
o Desmosine in non-helical segments, which is responsible for cross-linkage of the molecules to form a network that can alter its configuration when stretched.
The following diagrams illustrate the cross-linking of elastin to form a network
(a) in the relaxed state; (b) when stretched.

Fibrillin
Fibrillin is another extra cellular fibril that is often
associated with elastic fibres, but distinct from them.
Fibrillin:
o Forms fibrils 10 nm in diameter
o Is rich in cysteine and forms
numerous disulphide bonds
o Is present in
o Blood vessels and heart valves
o Suspensory ligament of lens
o Bones, tendons and ligaments
Marfan Syndrome results from a mutation of the fibrillin gene.
The clinical features of Marfans syndrome are:
o Skeletal:
Tall stature and arachnodactyly
o Cardiac: Aortic aneurysm; Heart valve incompetence
o Ocular: Subluxation of the lens
Proteoglycans are the major components of the amorphous extra cellular matrix
Proteoglycans:
o
Are large molecules forming the amorphous
matrix of connective tissues.
o  Consist of a protein backbone and
numerous polysaccharide side chains
Consist of a protein backbone and
numerous polysaccharide side chains
 Are rich in anionic radicals - COOH -
and - SO42-
Are rich in anionic radicals - COOH -
and - SO42-
o The large, branching molecules form complex aggregates that retain water
Properties of proteoglycans
Proteoglycans also give the amorphous matrix the
following properties:
o
High
viscosity
o Water retention
o Form a barrier to free diffusion of substances
o Some proteoglycans are
anticoagulants (e.g. heparin and dermatan sulphate)
o Some proteoglycans control cell
aggregation and growth during embryogenesis
Examples of proteoglycans
The following examples illustrate the functions of proteoglycans:
Chondroitin sulphate is present in large amounts in the matrix of hyaline cartilage, which is responsible for its hard, rubbery consistency, and a clear (hyaline) appearance. In experiments in which proteoglycans were broken down by enzyme treatment, cartilage lost its rigidity and turgidity.
Keratan sulphate is present in large amounts in the cornea, and is responsible for its transparency.
Other proteoglycans include
Dermatan sulphate is found in skin, blood vessels and heart and
Heparan sulphate is found in basement membranes, lung and arteries.
Hyaluronic acid is a non-sulphated proteoglycan, which is found in synovial fluid, skin and support tissues
Hyaluronidase breaks down hyaluronic acid reducing the viscosity and permeability of the matrix.
o It
is produced by some microorganisms to help them penetrate tissues
o It is used therapeutically as an ingredient of ointments such as Lasonil, which are applied to the skin to aid diffusion and absorption of haematomas.
Fibronectin and syndecan
Fibronectin and syndecan are proteins that are found in the amorphous matrix
of connective tissues, and provide attachment of cells to collagen. Each of these proteins consists of three
domains:
o A collagen - binding domain
o Two domains binding to proteoglycans
o A membrane-binding domain that binds
to the integral membrane protein integrin
The basal lamina consists of three layers:
o A lamina rara (or lucida), 60 mm thick, in contact with epithelial cells,
o A lamina densa 30-100 nm thick forms an intermediate layer
o A reticular lamina, which merges with the extra cellular matrix
It has four major constituents:
o Collagen
type IV - main component of reticular layer
o Heparan sulphate proteoglycan
o Laminin, the main component of the lamina rara, is a glycoprotein that binds the plasma membrane to proteoglycans.
o Entactin, a glycoprotein that binds to collagen type IV and to laminin
![]()


