Cell Interactions
Objectives
o Define the functions and properties
of cell recognition molecules
o Name different types of cell
adhesion molecules
o Explain how cell adhesion molecules
are involved in infections and the spread of cancer
o Outline the molecular structure of
different types of cell junctions
Recommended Reading
The World of the Cell. Becker WM, Kleinsmith LJ, Hardin J. 5th Edition.
Chapter 11
Cell-Cell Recognition and
Adhesion p302-306
Cell Junctions p306-314
The glycocalyx is a “fuzzy coat” on the external surface of the plasma membrane of cells. It is a carbohydrate-rich coat that consists of two indistinct parts:
o The attached glycocalyx consists of the branched carbohydrates of the glycolipids and glycoproteins of the plasma membrane
o The unattached glycocalyx consists of proteoglycans and glycoprotein of the extracellular matrix, which are rapidly washed away in vitro.
The glycocalyx has several different functions:
o Cell recognition
o Cell adhesion
o Protection
o Permeability barrier
Cell Recognition
And Cell Adhesion
During embryonic development undifferentiated cells arrange themselves into groups of functionally similar cells to form tissues. The cells recognise one another by means of a family of proteins called cell adhesion molecules (CAM).
Cell adhesion is necessary to keep the cells attached together. The same molecules perform both these functions of cell recognition and adhesion.
When cells from two different tissues are dissociated mechanically or by treatment with enzymes and then mixed in culture medium, the cells re-aggregate into different groups according to their origin. The cell adhesion molecules on the cell surface are the markers identifying the different cells.
Cell adhesion molecules may be involved in two types of interaction:
o Homophilic interactions where adhesion molecules on one cell interacts with identical molecules on the other cell
o Heterophilic interactions where an adhesion molecule on one cell functions as a receptor that binds to a different but specific molecule (known as the ligand) on the other cell.
There are two big
classes of cell adhesion molecules.
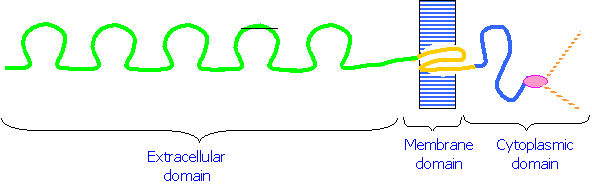
1. Cadherins are a class of cell adhesion molecules that are dependent on Ca2+ ions for their function. They are plasma membrane glycoproteins that consist of three domains:
1. An extracellular domain that binds to Ca2+ and interacts with similar domains on other cells
2. A hydrophobic domain that spans the plasma membrane
3. 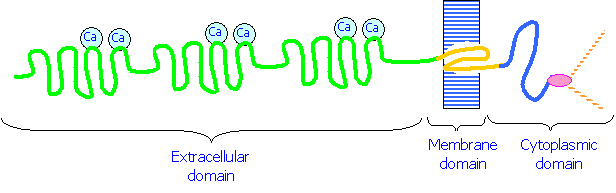 A cytoplasmic domain that binds to Catenins, molecules that in turn bind to the cytoskeleton.
A cytoplasmic domain that binds to Catenins, molecules that in turn bind to the cytoskeleton.
 Cadherins on one cell membrane interact with those on another cell in a zipper-like fashion. The bonds
between the cadherins can be removed by treatment with EDTA, which removes Ca2+ ions.
Cadherins on one cell membrane interact with those on another cell in a zipper-like fashion. The bonds
between the cadherins can be removed by treatment with EDTA, which removes Ca2+ ions.
Examples of cadherins include:
E- cadherin (on epithelial cells)
N- cadherin (on neurons)
P- cadherins (on placental cells)
2. CAMs are calcium-independent adhesion molecules. Like cadherins they are integral membrane proteins with membrane, cytoplasmic and extracellular domains. Their extracellular domains are
o Glycosylated
o Have 5 loops that resemble those of immunoglobulins - they belong the immunoglobulin super family (IgSF)
o Do not bind to Ca2+
The best-known examples of CAMs are N-CAM and L1-CAM, which are involved in formation of neuron contacts and grouping.
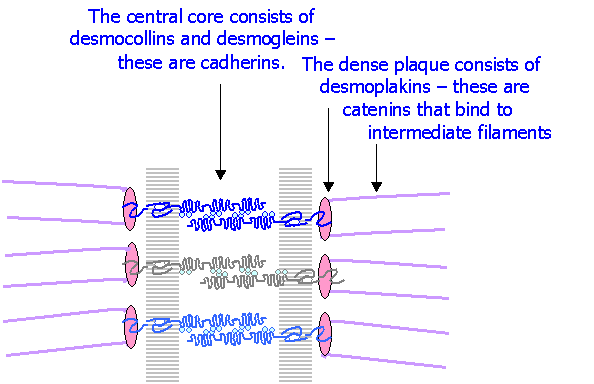
Desmosomes or Maculae adherentes are specialised sites that bind cells strongly to one another. They are circular areas of about 300 nm in diameter, and consist of:
o 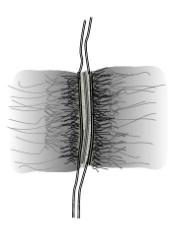 The apposed plasma membranes of two cells, separated by a 25-35 nm
gap
The apposed plasma membranes of two cells, separated by a 25-35 nm
gap
o A desmosome core occupying the intercellular gap
o A desmosome plaque on the cytoplasmic surfaces of the two membranes
o Intermediate 10 nm filaments attached to the desmosome plaques
High resolution EM shows connecting filaments that appear to traverse the plasma membranes.
The molecular structure of desmosomes
Cells may be dissociated from one another, without affecting their viability, by:
1. Treatment with EDTA, a chelating agent that effectively removes the Ca2+ disrupting the interaction between the cadherins.
2. Digestion with trypsin, which digests the extracellular domains of the cadherins.
Frequently a combination of trypsin and EDTA is used.
There are several clinical and practical applications of cell adhesion molecules:
Expression of cell
adhesion molecules precedes cell differentiation in the embryo.
Example: the primitive ectoderm is a layer of cells on the surface of the embryo, part of which differentiates into neuroectoderm that give rise to the central nervous system. Initially the primitive ectoderm expresses E-cadherin, but prior to differentiation some cells begin to express N-cadherin. This allows them to detach from the ectoderm and re-group to form the neural tube.
In cancer cells, changes or loss of cell adhesion molecules results in failure of the cells to adhere to one another, and begin to migrate away, spread and metastasise. Cancer cells transported in the blood stream metastasise preferentially in certain organs, depending on the cell adhesion molecules present
Many pathogens and parasites make use of recognition membrane proteins to colonize and bind firmly to mucosal surfaces. Otherwise they would be readily washed away. Bacteria such as E. Coli, Salmonella, Shigella, and Vibrio cholerae, all of which cause severe intestinal infections, have long projections (fimbriae) consisting of long protein chains of fibrillin that bind to the cell surface glycoproteins via specific sugars (e.g. mannose, fucose, galactose).
The microorganisms that cause gonorrhoea have a surface protein (pilin) that binds specifically to the glycoprotein coat that is present on the genital mucosae.
The malarial parasite (plasmodium falciparum) binds to the glycoprotein coat of erythrocytes prior to penetrating them.
Multiple sclerosis is caused by a deficiency in myelin-associated glycoprotein (MAG), a cell adhesion molecule similar to N-CAM that is responsible for adhesion between axons and Schwann cells that form the myelin sheath. This deficiency causes progressive loss of myelin.
Specialised cell junctions have special functions other than that of binding cells together.
Tight junctions are specialised junctions that occlude completely the extracellular space. They usually form a complete band around epithelial cells close to the luminal surface of the cells. They prevent fluids in the lumen (e.g. intestinal contents in the case of intestinal epithelium) from diffusing into the extracellular space between the cells.
Freeze fracture scanning EM shows that the band consists of a network of ridges. The ridges consist of rows of specific integral membrane proteins that form direct interactions with corresponding molecules on the corresponding molecules in the opposite cell membrane. The proteins do not project beyond the plasma membrane and thus effectively occlude the extracellular space. The specific membrane proteins have been identified and termed claudines and occludines.

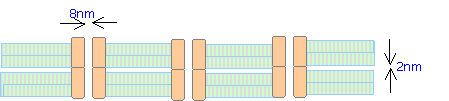 Gap junctions consist
of clusters of connexons, protein-lined channels that form communications
between adjacent cells and establish functional coupling between them. Each connexon consists of a hexamer
(complex of six) integral membrane protein molecules termed connexins that
surround an 8nm central pore. The
connexons on adjacent cells are aligned and the corresponding connexin
molecules link directly with one anther. The extracellular spaces are occluded
at the site of each connexon, but in the intervening regions the adjacent
plasma membrane are separated by a 2nm gap.
Gap junctions consist
of clusters of connexons, protein-lined channels that form communications
between adjacent cells and establish functional coupling between them. Each connexon consists of a hexamer
(complex of six) integral membrane protein molecules termed connexins that
surround an 8nm central pore. The
connexons on adjacent cells are aligned and the corresponding connexin
molecules link directly with one anther. The extracellular spaces are occluded
at the site of each connexon, but in the intervening regions the adjacent
plasma membrane are separated by a 2nm gap.
Membrane Domains of
Cells
Different regions of the plasma membrane of a cell vary in their structure and properties. As an example intestinal epithelial cells have the following membrane domains:
o An apical domain, characterised by microvilli and a thich glycoprotein coat;
o A lateral domain that borders on adjacent cells, further sub-divided into two domains, characterised by junctional complexes in the apical part and a wide intercellular space in its basal part.
o A basal domain that borders on, and is adherent to the basement membrane by hemidesmosomes.