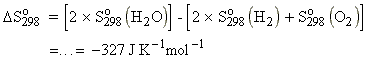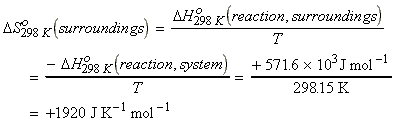Entropy and chemical processes
- DS in chemical reactions
- The use of DS to predict chemical reactivity
DS in chemical reactions
The standard reaction entropy,
is defined as the difference between the molar entropies of the pure, separated
products and the pure separated reactants, all substances being in their
standard states at the specified temperature (=298.15 K unless
otherwise stated). Thus for a generalised reaction we have:

The use of DS to predict chemical reactivity
For a reaction to occur, the second law of thermodynamics must be satisfied, i.e.:
Illustration:
Consider the reaction:
This means that:
Does this mean that since
the process does not occur at 298K (25oc)?
NO - The entropy condition that has to
be satisfied is ,
i.e. we need to have:
i.e.
NOTE: Although this is an excellent way to predict whether
a reaction is going to take place or not, it is not ideal to have to consider
the 'surroundings' all the time. It would be ideal to have a property which
would be solely dependent on the system. Such a property is called the
free
energy, and is considered in the next section.