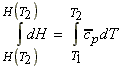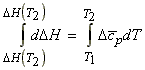Variation of
H with T
From
one may define cp, the heat capacity at constant pressure
as:
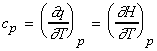
NOTE: More details on cv and cp
may be found here.
The variation of H with T
The variation of H with T may hence be studied by solving the differential equation above (i.e. by integration). We shall do this at two levels
(1) Elementary level
Assuming cp is constant in the temperature region of interest, i.e. for T in (T1, T2),(2) Higher level of complexity.
We may re-write eqn 1. As:
i.e.:
i.e.i.e.:
If T2 is very different from T1 than the approximation that cp is constant in the temperature region of interest cannot be made. Instead we approximate cp(T) as a polynomial in T, i.e.:
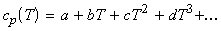
Note: (i) Any function can be expressed as a polynomial; (ii) For most cases, we assume that
gives a well enough description of cp.
i.e.:
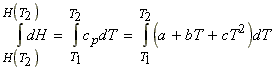
i.e.:
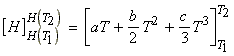
i.e.:
The variation of DH (or DHR) with T - applications to reactions.
In analogy to eqn. 1, we may write:
…………………… (KIRCHOFF's EQN.)
i.e.:
which integrates to:
This may be applied to compute DHR
for reactions at temperature T where T 298K (i.e. not standard). In
this case, cp refers to the reaction, i.e. for the reaction:
Applications
Example 1:
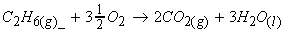
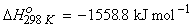
(i)
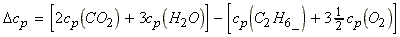
(ii)
Example 2:
This equation could also be useful, for example, for estimating
for T 298K, by letting T2 = T and T1
= 298K, i.e.: