![]()
 Museum
Museum
![]()
SM090
Callendar’s apparatus
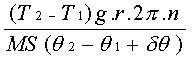
In a typical
experiment on apparatus SM090:
Mass of water
in calorimeter was 75 grams
Mass of
calorimeter 286 grams
Specific heat
of copper, 0.1 calories/gram/deg C
Radius of
calorimeter 3.80 cm
T2 =
1000 g, T1 = 125 g
Duration of
temperature rise 5 minutes
Initial
temperature = 26.06 deg C
Final
temperature = 28.42 deg C
Number of
revolutions 550
Rate of cooling
0.046deg C
Cooling
correction from above = 0.115 deg C
So corrected
temperature rise is 2.48 deg C
Using the
formula the mechanical equivalent of heat = 43.8 ergs per calorie
Since 1J = 1x107
ergs, therefore mechanical equivalent of heat is 4.38 Joules/calorie
The standard result is 4.16 Joules/calorie.
![]()
