
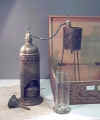
SM140
Distillation Apparatus for Alcohol
Content circa 1900
Heat
The liquefaction or condensation of a vapour is the change from the
vapour to the liquid state. Condensation
is caused by one of three factors:-
cooling, compression or chemical reaction.
For the first two, the vapour must be saturated while the last
method can bring about the liquefaction of even rarefied vapours.
An example is the absorption by certain salts of aqueous vapour
from the atmosphere.
Distillation is the separation of a volatile liquid from a substance
that it holds in solution, or the separation of a mixture of two
volatile liquids.
Liebig’s condenser consists of a glass tube fitted in a jacket through
which cold water is passed. The
liquid to be distilled is contained in a retort, the neck of which is
connected to the condenser tube.
The liquid is heated and the distillate is collected as drops
from the cold end of the condenser.
The process of distillation is used to determine the alcoholic value of
wines in a specially modified condenser.
The distillation process removes all solids and produces a
distillate containing all of the alcohol and part of the water.
The liquid part is reconstituted as before and the specific
gravity gives an accurate measure of the alcohol content.

![]()
 Museum
Museum
![]()
![]()